How to Remove Chloramines From Drinking Water
July 8, 2022 10:05 am Leave your thoughts
Although Australians enjoy high-quality water by international standards, our water supply can still contain a variety of contaminants.
This can include chemicals, disinfection by-products, sediment, heavy metals, rust, and much more.
Chlorine is an obvious point of concern for most Australians, as it adds a very obvious bad taste and odour to water. The introduction of chlorine can also create trihalomethanes, a potentially dangerous chemical.
To reduce the negative impact of chlorination, some Australian water authorities have begun introducing chloramines into their water supplies.
Although chloramination doesn’t introduce high levels of trihalomethanes like chlorination does, it does have its own set of issues.
In this guide, I’m going to share the problems associated with chloramination and how you can filter chloramines from your water.
If you have any questions about chloramine removal, please give Clarence Water Filters a call on 0266468565.
Contents
Chlorination of Australian Drinking Water
What Are Chloramines?
How Long Have Chloramines Been In Use?
Are Chloramines Safe To Drink?
What About Chloramine Gas?
How To Remove Chloramines From Water
– Active Carbon Filtration
– Catalytic Carbon Filtration
– Kinetic Degradation Fluxion (KDF)
– Reverse Osmosis
Best Water Filters For Chloramine Reduction
– 10″ x 2.5″ WC04 Carbon Block
– Whole House Water Filter System For Chlorine and Chloramines
– 10″ x 2.5″ CRC2510-P5 Chloramine Reduction Filter
– Omnipure L5515 Carbon Block Filter
– AMB/10 and DFX-CB-10 Filter Set
Frequently Asked Questions

Chlorination of Australian Drinking Water
Most Australian water authorities currently use chlorine to disinfect drinking water. That’s because chlorine is extremely effective at killing pathogens including bacteria, viruses, and algal spores.
Chlorination also provides some residual protection, which means the water can remain resistant to contaminants for several days. It is a highly scalable solution that is easy to implement.
Unfortunately, there are several downsides associated with the use of chlorine for water disinfection, including:
- Horrible taste and smell
Chlorine can introduce a sour chemical taste and smell to water, which many people find unpalatable. - Disinfection by-products
When chlorine interacts with decaying organic matter it can create toxic disinfection by-products (DBPs) including trihalomethanes and haloacetic acids (HAAs). Trihalomethanes are a group of chemicals linked to colon and rectal cancer while HAAs are linked with bladder cancer. The World Health Organisation has written extensively about the risks associated with chronic DBP exposure. - Relatively low protection against protozoa
Although chlorine works well on viral, algal, and bacterial contaminants, it isn’t always good against protozoa. - Lower disinfection effectiveness in turbid waters
If the water has high levels of turbidity (haziness or cloudiness), chlorine will be much less effective. This can be particularly problematic after a weather event like flooding rains, which can increase water turbidity.
To overcome the problems associated with chlorination, many water authorities have begun using chloramines.

What Are Chloramines?
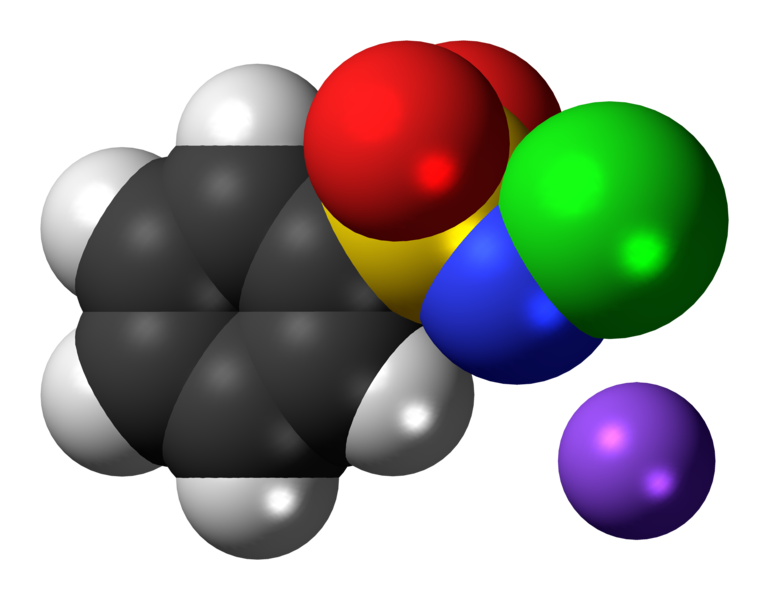
Chloramines are disinfectants used to treat drinking water. They are created by adding ammonia to water that contains chlorine.
The most common forms of ammonia used for chloramination in Australia are liquefied anhydrous ammonia and aqueous ammonia.
There are several forms of chloramines including monochloramine (NH2Cl), dichloramine (NHCl2) and trichloramine (NCl3). The chemical reactions used to create these chemicals being:
HOCl + NH3 → NH2Cl + H20 (monochloramine)
HOCl + NH2Cl → NHCl2 + H20 (dichloramine)
HOCl + NHCl2 → NCl3 + H20 (trichloramine)
The type of chloramine that is formed when ammonia is added to water will depend on the water’s pH. If the pH is less than 4.4, trichloramine is formed. If it is between pH 4.4 – 6.0, then dichloramine is formed.
If the water is neutral (7 pH) or higher, monochloramine will be formed. Because most municipal water supplies in Australia are treated to have a pH above 7, monochloramine is most common form of chloramine.
Water authorities are increasingly using chloramines in the water supply as it has several advantages:
- Improved residual protection
Chloramines will persist in water for a longer period than active chlorine, which is useful when sending water over long distances. The longevity of chloramines means water treatment plants can be located further from suburbs and water can be held in storage tanks or water pipes for longer periods without pathogens developing. - Reduced impact on taste
Water authorities claim that chloramines have less impact on the taste of water compared to chlorine. However, many people find that water treated with chloramines still has a metallic or tangy taste. - Fewer disinfection by-products
Compared to chlorine, Chloramines will produce fewer toxic by-products when they interact with other chemicals and decaying organic matter. However, they can still generate some potentially toxic substances including Nitrosamines and Iodoacid (more on these chemicals shortly). - Can remove biofilms
A biofilm is a slime that contains a collection of organic and inorganic, living and dead material. It can form within water pipe networks, potentially impacting the safety of drinking water. The persistence of chloramines makes them ideal for removing biofilms that have accumulated in water infrastructure.
It’s worth noting that there are a few downsides which make the use of chloramination as a primary disinfectant rare:
- More time is required for chloramines to be effective
One problematic issue for water authorities is that chloramines take much longer to work compared to active chlorine. This is why chloramines are often used as a method of secondary disinfection, in combination with active chlorine. - Smells terrible
Although chloramines have less of an effect on the taste of water compared to chlorine, they can have a dramatic impact on smell. Water with high levels of chloramines begins to smell like a heavily chlorinated swimming pool. - Expensive to use
The other issue for water authorities is that chloramination is a more expensive and complicated method of sanitisation as the amount of ammonia being added must be carefully measured. As a result, it is mostly used in situations where long-term protection is essential or chlorination disinfection by-products like trihalomethanes have been a problem in the past. - Less effective against certain pathogens
Chloramine isn’t as good as chlorine at reducing pathogens like E Coli from water. This is the main reason why water authorities normally let chlorine do its work before adding ammonia to form chloramines. - Corrosion
When chloramines are added to town water, it can trigger several chemical reactions. If the water has lower pH and alkalinity, it can make the water mildly corrosive to metal. Nitrification can also occur when the ammonia in the water converts into nitrates. The nitrates can corrode copper plumbing in homes and cause pinhole leaks. Drinking water that contains dissolved copper can be quite dangerous. - Toxic for some animals
Both chlorine and chloramines are toxic to reptiles, aquatic animals, and amphibians. This is why it is essential that you filter out chloramines if you have an aquarium or keep reptiles or fish at home. - Unsuitable for hemodialysis patients
Water containing chloramines is unsuitable for use in dialysis fluid as it can enter the blood of patients and cause haemolytic anaemia.

How Long Have Chloramines Been In Use?
Chloramination has been used in the United States, Britain, and Canada for over 90 years. However, it has been less common in Australia until the last few decades.
As suburban sprawl has become more widespread in Australia, water authorities have increasingly turned to chloramines. Their goal being to achieve improved residual protection of water as it is piped long distances to homes and businesses.
Are Chloramines Safe To Drink?
Australian municipal, state, and federal authorities have stated that chloramines are safe for human consumption, as long as they remain within specific limits.
In the United States, the Environmental Protection Agency has said that water can contain up to 4mg/L of chloramines and still be safe to drink and bathe in.
However, there is a growing body of evidence which suggests that the disinfection by-products (DBPs) created during chloramination may be dangerous. Two of the most concerning DBPs are:
- Nitrosamines
Nitrosamines are organic compounds with the chemical structure R2N−N=O. They can be created when dichloramine interacts with nitrogen precursors in the water supply.
Researchers have discovered that nitrosamines are toxic in animal studies. They are strong carcinogens that can produce cancer in many organs and tissues including the liver, kidney, lung, brain, bladder, stomach, esophagus, and nasal sinus.
Research is ongoing to understand the level of Nitrosamines in water treated with chloramines and whether it poses a significant threat to humans.
- Iodoacid
There have been several research papers which indicate that Iodoacid (IA) represents a new class of highly toxic drinking water contaminants that can be created during chloramination.
They have discovered both cytotoxicity and genotoxicity in animal tests. Researchers are still working to understand if this chemical presents a threat.
What About Chloramine Gas?
When water containing high levels of chloramines is heated, toxic chloramine and DBP vapours can be created. These gasses can accumulate in indoor environments fairly quickly and may also pose a threat to your health. Some of the symptoms you may experience when exposed to high levels of chloramine gas include:
- Burning, watery eyes
- Difficulty breathing
- Coughing and wheezing
- Nausea
- Chest pain
- Fluid build up in the lungs

How To Remove Chloramines From Water
Chloramines cannot be removed by boiling, distilling, or by standing uncovered. This is due to the fact they are complex molecules which persist for a long time in most environments.
Fortunately, chloramines can be removed from drinking water through filtration. However, they are much harder to remove from water compared to common contaminants like sediment and chlorine.
Here are the best options for chloramine removal:
Active Carbon Filtration
Activated carbon is carbon which has been modified to create millions of additional pores on its surface. It can be made from many types of carbon, however crushed coconut shells and coal are the most common options.
As water flows through the carbon, contaminant molecules adhere to the carbon’s pores. This is how it traps chlorine, trihalomethanes, petrochemicals, volatile organic compounds and other chemical compounds.
For activated carbon to trap chloramines, it must have plenty of contact time – usually 3 to 4 times more contact time than is needed for chlorine removal. This is due to the fact that chloramines have exceptional chemical stability.
Contact time can be improved by having a slower flow rate, using a larger amount of carbon, or installing a dense carbon block, which is tightly compacted powdered carbon.
The carbon block will contain more carbon than a Granular Activated Carbon filter of the same size which means more surface area for chloramine removal.
A high-quality 10″ carbon block like the WC04 will remove virtually all chloramines from the water.
This means some filters will be adequate for chlorine removal but not have a sufficient amount of carbon to remove chloramines at the same flow rate (More on the activated carbon filters capable of reducing chloramines shortly).
If you need to use loose carbon to fill a whole house water filter cartridge or place into a vessel, we would recommend the use of a ‘surface enhanced’ activated carbon like catalytic carbon.
Catalytic Carbon Filtration
Catalytic carbon, also known as Odour Reducing Carbon (ORC) has been chemically processed so it has more pores compared to standard activated carbon.
Having additional pores means it can adsorb and catalyse (break apart) persistent chemical compounds like chloramines much better than activated carbon on its own.
The additional pores also mean that catalytic carbon needs less contact time to work, which helps it support faster flow rates. In other words, it can reduce chemicals at a faster rate than activated carbon and hold more contaminants.
Kinetic Degradation Fluxion (KDF)
KDF is a blend of high-quality copper and zinc. As oxygenated water flows through the media, an electrochemical process called redox occurs, which causes certain contaminants to lose or gain electrons.
Chlorine, for example, loses electrons to change into chloride — a harmless electrolyte. KDF will reduce chloramine by taking the molecule apart, changing the chlorine molecule to chloride and leaving the ammonia behind.
KDF alone won’t remove a high level of chloramines. However, it greatly enhances the function of activated or catalytic carbon, leading to much better results.
KDF85 is the most effective form of KDF for reducing chloramines, as this research paper demonstrates. The only downside of using KDF85 is that it can be expensive. As a result, many home owners opt for systems that use catalytic carbon on its own.
Reverse Osmosis
Chloramines can also be reduced by reverse osmosis systems. These systems use several filters and a very fine membrane to remove most of the contaminants in water.
Unfortunately, there are several downsides associated with using reverse osmosis, including high levels of water wastage, the removal of beneficial minerals from the water, and high running costs.
How Do I Know If Chloramines Are In My Water?
They are obligated to publish test results and to disclose the level of chlorine/chloramines in your drinking water.
Alternately, if you are really concerned about the safety of your water, you could have it professionally tested.
While there are consumer-level test kits for reading chloramine levels in water, they are typically unreliable.
How Long Can Chloramines Last in Water?
But to provide you with some general numbers — If you had 40 litres of undisturbed water with 1ppm chloramines, it would take approximately 170 hours for the chloramines to dissipate. If the water is being circulated, that figure is cut to 70 hours.
Can You Remove Chloramines by Boiling the Water?
However, it would be “extremely” slow and would negatively impact the flavour of the water.
Boiling releases a tiny amount of dissolved chloramines as gas. The amount removed from the water is a tiny fraction and it would be impossible to remove all of the chloramines via this method.
Why Isn't Activated Carbon As Effective At Removing Chloramines?
The chloramine molecule is quite complex and difficult to catalyse (break apart). As a result, chloramines can move past the pores in activated carbon without becoming stuck or torn up in the same way that active chlorine particles do.
Activated carbon will still be partially effective at remove chloramines, but it will take a lot more carbon for it to reach high removal rates.
Catalytic carbon has been processed to have additional pores on its surface. As a result, it can break down chloramines much faster and achieve high removal rates. The alternative is to use a carbon block which has been tested and found effective at chloramine removal.
Will Reverse Osmosis Remove Chloramines?
A reverse osmosis system uses multiple water filters and an ultra-fine reverse osmosis membrane to remove the majority of contaminants.
It’s important to note that the membrane won’t remove any chloramines. It will be completely up to the quality of the carbon pre-filters in the unit. If you want to be completely sure that chloramines are removed, use a high quality carbon block water filter in your RO system.
Because RO systems have an extremely slow flow rate, you will ensure there is plenty of contact time between the carbon and the water, which generally leads to excellent removal rates.
Best Water Filters For Chloramine Reduction
WC04 0.45 Micron Carbon Block
The WC04 is a double open ended 10” x 2.5” carbon block filter which suits standard 10” QMP and Aquapro housings.
It is an American made product which reduces a wide range of contaminants including chlorine, chloramines, disinfection by-products (trihalomethanes etc), heavy metals and sediment.
The WC04 is often used in under sink and benchtop filtration systems. It is an affordable option which delivers excellent results while retaining a high flow rate.
Product Links:
Single Under Sink Filter System With WC04
Twin Under Sink System With WC04
Benchtop Water Filter With WC04
Multipure WC04 Replacement Filter
Town Water Chlorine & Chloramine Twin Whole House Water Filtration System
This is a complete whole of house system designed to be placed on the incoming water line from the mains. It filters 100% of your incoming water, before it reaches any of the water-consuming appliances or water outlets in your property.
It is a twin system with a sediment filter in the first cannister and a refillable KDF/Catalytic carbon chemical reduction filter in the second.
The combination of KDF55 and Catalytic Carbon does a wonderful job of reducing chloramines and other chemical contaminants. The large filter size helps the unit filter out contaminants at a fast flow rates.
Product Links:
Whole Of House Water Filter For Town Water Chlorine/Chloramines
Chloramines and Chlorine Reduction Filter CRC 2510-P5
The CRC 2510-P5 is a double open ended 10” x 2.5” filter designed for chlorine and chloramine reduction. It has a sediment wrap surrounding a 0.5 micron carbon block. Effective flow rate of up to 3.6 Lpm.
Links:
Chloramines and Chlorine Reduction Filter CRC 2510-P5
Omnipure L5515 Carbon Block Filter
Omnipure is one of the world’s leading filter manufacturers. They offer a wide range of products for both commercial and residential applications.
The L5515 is a fine carbon block filter which can remove chloramines, chlorine, volatile organic compounds, petrochemicals and many other contaminants. It uses the L-series
L-series filters require the use of a special filter head. This filter is simply screwed in like a bayonet lightbulb.
Product Links:
Omnipure L5515 Carbon Block Filter
AMB/10 and DFX
The AMB/10 and DFX combination is a great choice for anyone interested in removing fluoride as well as chloramines. These filters are both standard double open ended, 10” x 2.5” filters which work in standard QMP housings.
The DFX-CB-10 is the first filter in the series. It is a 10 micron carbon block filter which does a great at reducing chemicals, sediment and other contaminants. It acts as a pre-filter, protecting and improving the performance of the AMB/10.
The AMB/10 is a triple stage system which reduces Fluoride up to 92% at 1900 litres, Chloramines up to 99% at 13300 litres, Chlorine up to 99%, Lead up to 99% at 13300 litres, Heavy Metals up to 98%, and Pharmaceutical Componds up to 95%.
Twin Fluoride and Chloramine Reduction Filter Set
Wrapping Up
The use of chloramines looks set to increase over the coming years. Fortunately, filtration is an effective wat to remove chloramines from your water. If you need any more chloramine removal filter options, get in touch today:
Clarence Water Filters Pty Ltd
Phone: 02 6646 8565
Email: sales@clarencewaterfilters.com.au
Tags: Buyers Guide, Chloramine, Chloramine Reduction, Chloramines, ContaminantsCategorised in: Buyer's Guides, Contaminants
This post was written by Paul





